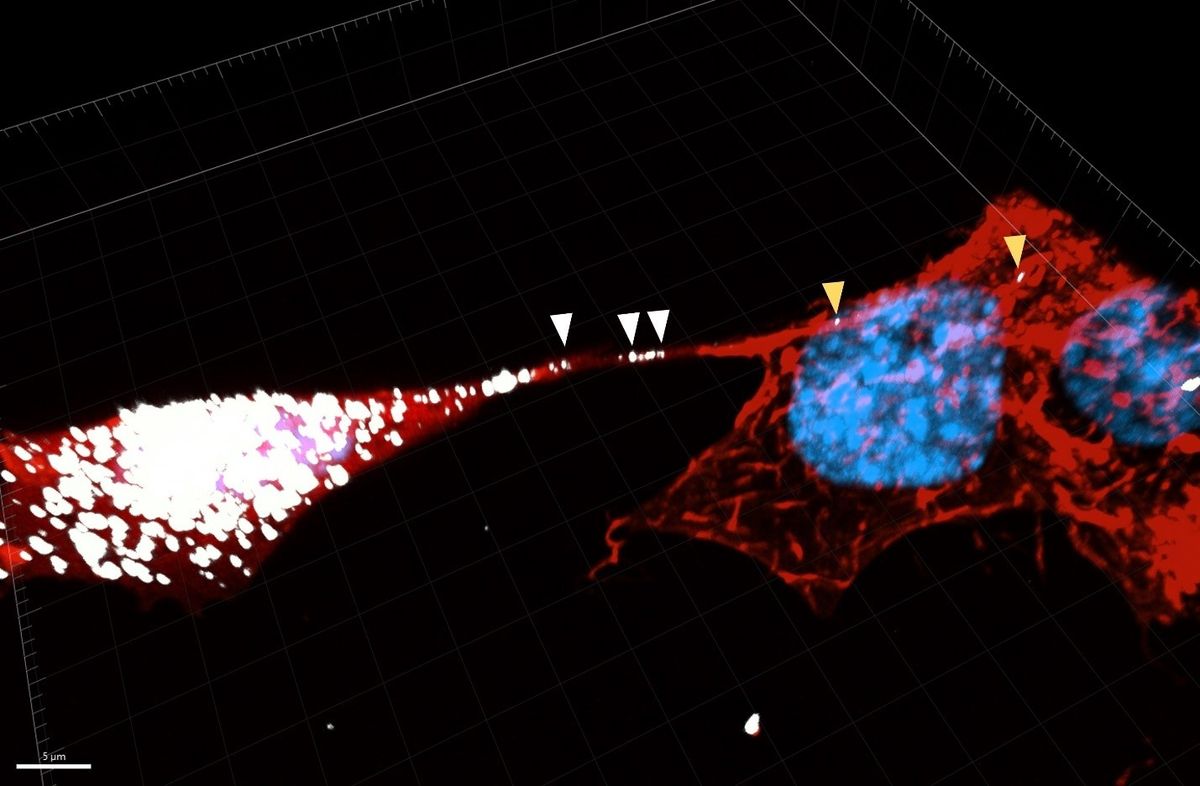
One of the biggest controversies in neuroscience dates back to 1906, when Camillo Golgi and Santiago Ramón y Cajal argued their opposing views on brain structure.1 Golgi hypothesized that brain cells are physically connected and, consequently, the cells function as one entity. Conversely, Cajal hypothesized that each brain cell functions independently, but communicates with each other. While Cajal’s theory has formed the basis of neuroscience as we know it, Golgi’s theory was not completely incorrect. In 2004, Hans-Hermann Gerdes’ group from the University of Heidelberg discovered open-ended, membrane-enclosed channels between neuron-like cells, called tunneling nanotubes (TNTs).2 However the discovery of TNTs has not gone without its own controversy.
“People didn't believe these structures. They couldn't believe that cells would communicate through tunnels. This goes against the dogma of the cell as an individual entity,” said Chiara Zurzolo, the head of the Membrane Traffic and Pathogenesis Unit at the Institut Pasteur. Decades of research later, researchers have shown that TNTs permit the exchange of ions, organelles, and protein aggregates between a variety of cell types, such as neurons and astrocytes. While investigating if microglia, the resident immune cells of the brain, and neurons communicate through TNTs, Zurzolo and her team uncovered a protective role for microglia in neurodegenerative diseases and published these findings in Cell Death and Disease.3
Zurzolo became interested in microglia because they internalize and degrade toxic protein aggregates and, therefore, can prevent the build up of aggregates within the brain. As aggregate accumulation is characteristic of many neurodegenerative diseases such as Alzheimer’s disease and Parkinson’s disease, and ultimately results in neuronal death, this suggests that microglia protect the brain from neurodegeneration.4 However, microglia can also directly or indirectly cause neuronal death, which could exacerbate neurodegeneration. “It’s not clear whether microglia are…facilitating the progression of these diseases or trying to block the progression…to save the neuron,” said Zurzolo.

Zurzolo’s team first determined if functional TNTs form between neurons and microglia. They treated a neuronal cell line with α-Synuclein (α-Syn), a protein commonly aggregated in Parkinson’s disease, and co-cultured the neurons with microglial cells. The team observed α-Syn aggregates moving from aggregate-burdened neurons to microglia, which they think reduces neuronal cell stress and likely permits aggregate degradation by microglia.5 When microglia were exposed to α-Syn before the researchers co-cultured them with neurons, the majority of the burdened microglia did not transfer aggregates to the neurons, which suggested that microglia protect neurons from becoming exposed to aggregates.
In addition to aggregate transfer, the researchers discovered another way that microglia help α-Syn-burdened neurons. As aggregate accumulation can result in mitochondrial damage in neurons, Zurzolo’s team assessed if mitochondria are transferred from microglia to neurons through the TNTs. The team incubated neurons with α-Syn, labeled mitochondria in microglial cells, and cultured the cells together. They discovered that mitochondria from microglia were preferentially transferred to unhealthy, aggregate-filled neurons over healthy neurons. Ultimately, Zurzolo thinks this exchange between neurons and microglia can relieve the pressure placed on unhealthy neurons by permitting the microglial-mediated destruction of aggregates and providing the neurons with healthy mitochondria to overcome the aggregate-mediated mitochondrial damage.
For TNTs, we don't know almost anything…but because it's new…[it’s] a million times more exciting.
–Chiara Zurzolo, Institut Pasteur
“I think [the study] consolidates what we know about microglia being important to remove toxic aggregates,” said Marie-Ève Tremblay, an associate professor at the University of Victoria, who was not involved in the study. “I think it fits with the overall known functions of microglia while explaining the new ways by which these functions are achieved.”
Zurzolo is hopeful that further research into TNTs can shed more light on the communication between healthy and unhealthy cells in the brain, as well as the importance of TNTs to development and aging. Moreover, TNT-mediated communication is relatively unexplored in comparison to other intercellular communication mechanisms, including receptor-mediated endocytosis, which makes further insight into this mechanism valuable. “For TNTs, we don't know almost anything…but because it's new…[it’s] a million times more exciting,” Zurzolo said.
References
- Zurzolo C. Tunneling nanotubes: Reshaping connectivity. Curr Opin Cell Biol. 2021;71:139-147. doi:10.1016/j.ceb.2021.03.003
- Rustom A, Saffrich R, Markovic I, Walther P, Gerdes HH. Nanotubular highways for intercellular organelle transport. Science. 2004;303(5660):1007-1010. doi:10.1126/science.1093133
- Chakraborty R, Nonaka T, Hasegawa M, Zurzolo C. Tunnelling nanotubes between neuronal and microglial cells allow bi-directional transfer of α-Synuclein and mitochondria. Cell Death Dis. 2023;14(5):1-12. doi:10.1038/s41419-023-05835-8
- Menzies FM, Fleming A, Caricasole A, et al. Autophagy and Neurodegeneration: Pathogenic Mechanisms and Therapeutic Opportunities. Neuron. 2017;93(5):1015-1034. doi:10.1016/j.neuron.2017.01.022
- Scheiblich H, Dansokho C, Mercan D, et al. Microglia jointly degrade fibrillar alpha-synuclein cargo by distribution through tunneling nanotubes. Cell. 2021;184(20):5089-5106.e21. doi:10.1016/j.cell.2021.09.007