ABOVE: © ISTOCK.COM, koto_feja
Doctors treat epilepsy with anticonvulsants to control seizures, but some patients do not respond to these first-line therapies. For patients with drug-refractory epilepsy (DRE) whose seizures persist after treatment with two or more anticonvulsants, clinicians must surgically remove part of the brain tissue to cure the disease.
When first-line medicines fall short, scientists examine the molecular mechanisms of a disease to understand why and to develop alternatives. At Duke-NUS Medical School and KK Women’s and Children’s Hospital, clinicians and researchers teamed up to investigate how inappropriate proinflammatory mechanisms contribute to DRE pathogenesis. This work builds on evidence that inflammation is associated with epilepsy. For Derrick Chan, a clinician scientist at KK Women’s and Children’s Hospital, this research is an extension of his clinical work. “This direction became really important because we were looking for a less invasive way to try to help all the children with drug resistant epilepsy,” he said.
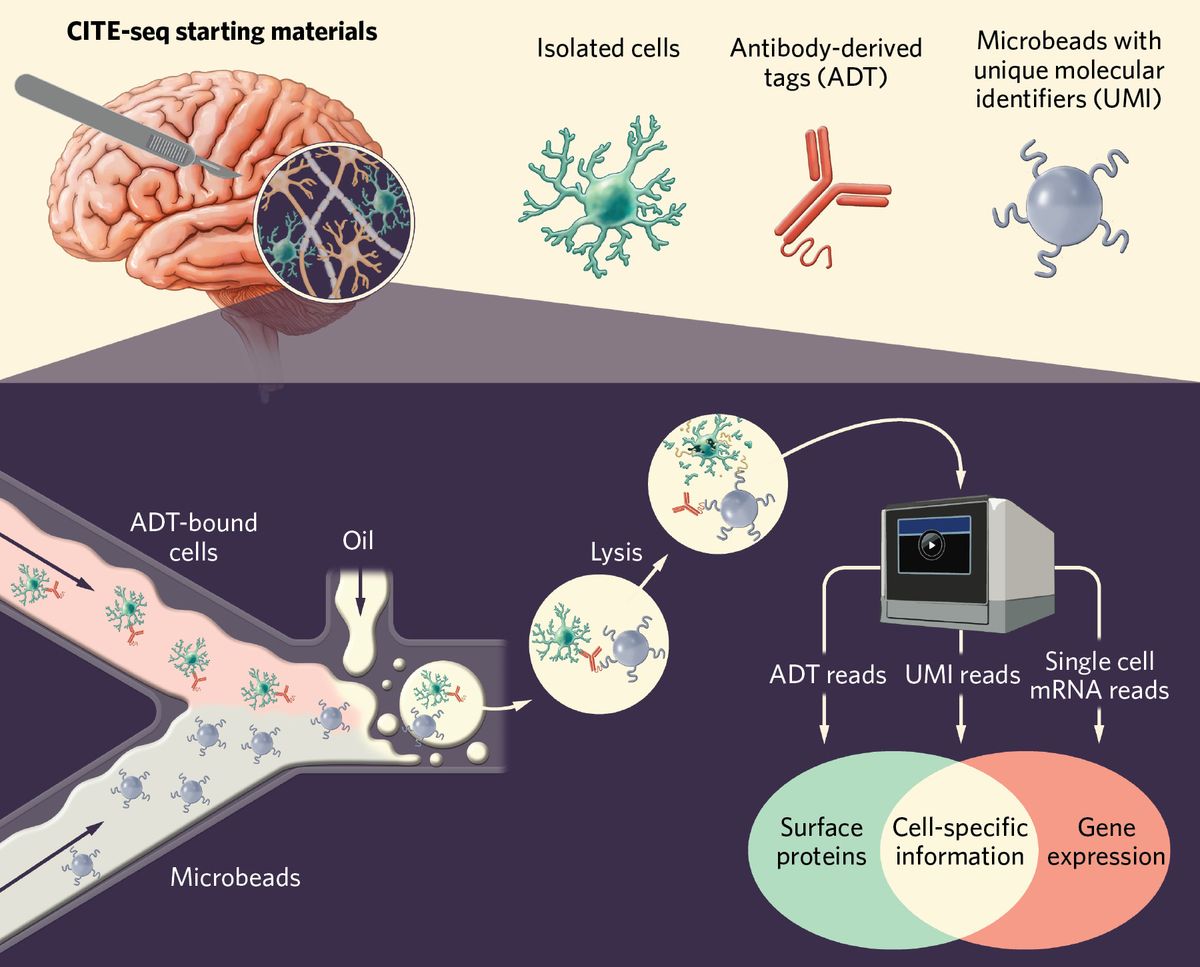
Chan and his team partnered with the immunology research group of fellow physician scientist, Salvatore Albani. In a study published in Nature Neuroscience, Chan and Albani described their efforts to understand the immunologic factors that contribute to DRE pathology. The researchers used a single-cell sequencing technique called cellular indexing of transcriptomes and epitopes by sequencing (CITE-seq) to examine surgically resected epileptic tissue from patients.
They identified cell-specific functions at single-cell resolution and differentiated resident cells from infiltrating immune cells. The researchers found that the DRE microenvironment includes activated microglia and other proinflammatory immune cells. “We had not expected these interactions between microglia and other immune cells, and then how these microglia become kind of a pivot to attract all of the immune cells by starting this proinflammatory milieu inside the brain,” explained Pavanish Kumar, coauthor of the study.
Previous work from Albani and Chan underscored how systemic inflammation contributes to epilepsy pathogenesis, which prompted them to investigate the local inflammatory signature in brain tissue. It can be a challenge to study brain tissue, and as Albani pointed out, access to samples is one limitation of this study. “It’s not as simple to get brain tissue from live patients… so numbers are small,” he explained. The researchers emphasized that future work will involve animal models to establish a robust foundation to build on.
“It’s definitely a big jump forward in our field,” said David Henshall, a professor of physiology and medical physics at Royal College of Surgeons, who was not involved in the study. “Perhaps we can now, based on this study, design a treatment or combination of treatments that may be more precise than what has been tried so far.”